INTRODUCTION
Many people use the terms horns and antlers inter- changeably. They both grow from an animal’s forehead and both are used for the assertion of dominance, providing de- fence and attracting the mates.
Horns and antlers have a different evolutionary history and are made from somewhat different precursors. Horns evolved primarily in the family of cloven-hoofed ruminant mammals, or ‘bovids’ such as sheep, goats, cows and bison. Antlers, on the other hand, evolved in a different family of ruminants called ‘cervids’, which includes all deer, elk, moose and caribou (or reindeer). In addition, another notable difference between horns and antlers is that the horns are unbranched but antlers are branched with multiple points. Horns are made of keratin, like your fingernails (more accu- rately they consist of a bony core and a keratinized sheath), they grow throughout the entire lifetime of an animal and they are permanent and are never shaded, Antlers, on the other hand, are made of bone and they are shaded every year and in the fall, a whole new set grows back in time for the mating season. The antler cycle is regulated by the testicular cycle [1, 2]. Horns consist of a covering of keratin (and some other proteins) surrounding a core of live bone. True horns are found mainly among the ruminant artiodactyls, in the families Antilocapridae (pronghorn) and Bovidae (cattle, goats, antelope etc.). Though horns are never branched, they do vary in shape and size from species to species.
The horns grow in a completely different way as com- pared to that of the antlers. Neither the sheath nor the core is ever shed and in many species, the horns never stop growing. Horn cores begin as a small bony growth under the skin, over the skull and, in the subcutaneous connective tissue. They are not attached to the skull and are known as ‘ossicones’. They possess their own centres of ossification and fuse secondarily to skull bones. In members of the family Bovidae, horns develop from or over the frontals.
Similar to antlers, horns are often used by the males for the purpose of fights and displays during the breeding sea- son. In general (but with many exceptions, including saiga), horns are present in both sexes of a species with a relatively large body-size but are absent in the females of smaller spe- cies. This is probably due to the fact that larger species are more likely to engage into a fight, whereas smaller species tend to run and/or hide. In the species where members of both the sexes have horns, some degree of sexual dimor- phism is usually observed as a rule. The horns of the males are usually thicker at the base and are able to withstand more force, whereas the horns of the female members are straighter and thinner, which may make them better suited for stabbing (defensive weapons).
The horns of the Rhino differ from the ‘true’ horns with regard to the core or sheath, both of which are absent in rhino. They are made up of a multitude of epidermal cells and bundles of dermal papillae – extensions of the dermis. Cells from each papilla form a horny fibre similar to a thick hair. These fibres, which are held together by the mass of epidermal cells, are not true hair, because true hair grows from the follicles that extend into the dermis whereas rhino horns grow from the dermal papillae which extend into the horn. The Rhino horns commonly have a posterior curvature and are situated over the nasal bones in all the rhino species; the second horn, found in species with two horns, lies over the frontal bones.
The antler and horn-bearing animals are deeply rooted in the human culture and partly, the observed functional sig- nificance of horns and antlers in the survival and reproduc- tion of these animals has facilitated their popular use in tradi- tional medicine.
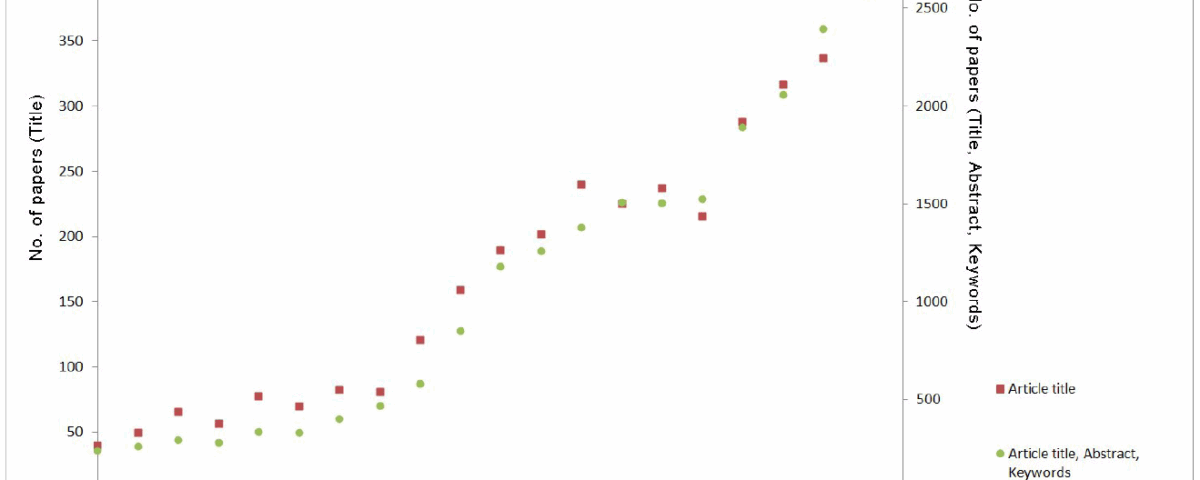
Traditional Chinese medicine (TCM) has been widely used for thousands of years, both to prevent and to treat vari- ous diseases, and a renewed interest in TCM is demonstrated by the tenfold increase in the number of published articles on the topic in the last 20 years (Fig. 1).
Probably the most famous use of horn in the TCM is that of the Rhino, which has been used for over 2000 years. It is strongly believed that it has a beneficial influence on human. Some recent studies propose that compounds derived from the rhinoceros horn are effective in the treatment of hyper- thermia, whereas some studies have researched its antipy- retic properties; however the conclusions drawn by these studies remain contradictory [3-7].
The horn of the saiga antelope is also frequently used in TCM. Its popular name in Chinese is ‘Lin Yan Jiao’ and the pharmaceutical name is Cornu Antelopis. It is as famous and valuable as musk, pilose antler (Lurong) and rhino horn, the most-renowned medicinal material of animal origin used in TCM. The textual record of the use of ‘Lin Yan Jiao’ may date back to the ‘Shengnong Bencao’ (Divine Husbandman’s Classic of the Materia Medica), 2000 years ago. In the early 1990s, in an effort to reduce the loss of rhinoceros to poach- ing, conservationist organizations advocated the use of the horn of the saiga antelope in TCM. By 2001, however, the saiga also joined the rhinoceros on the list of endangered species [8].
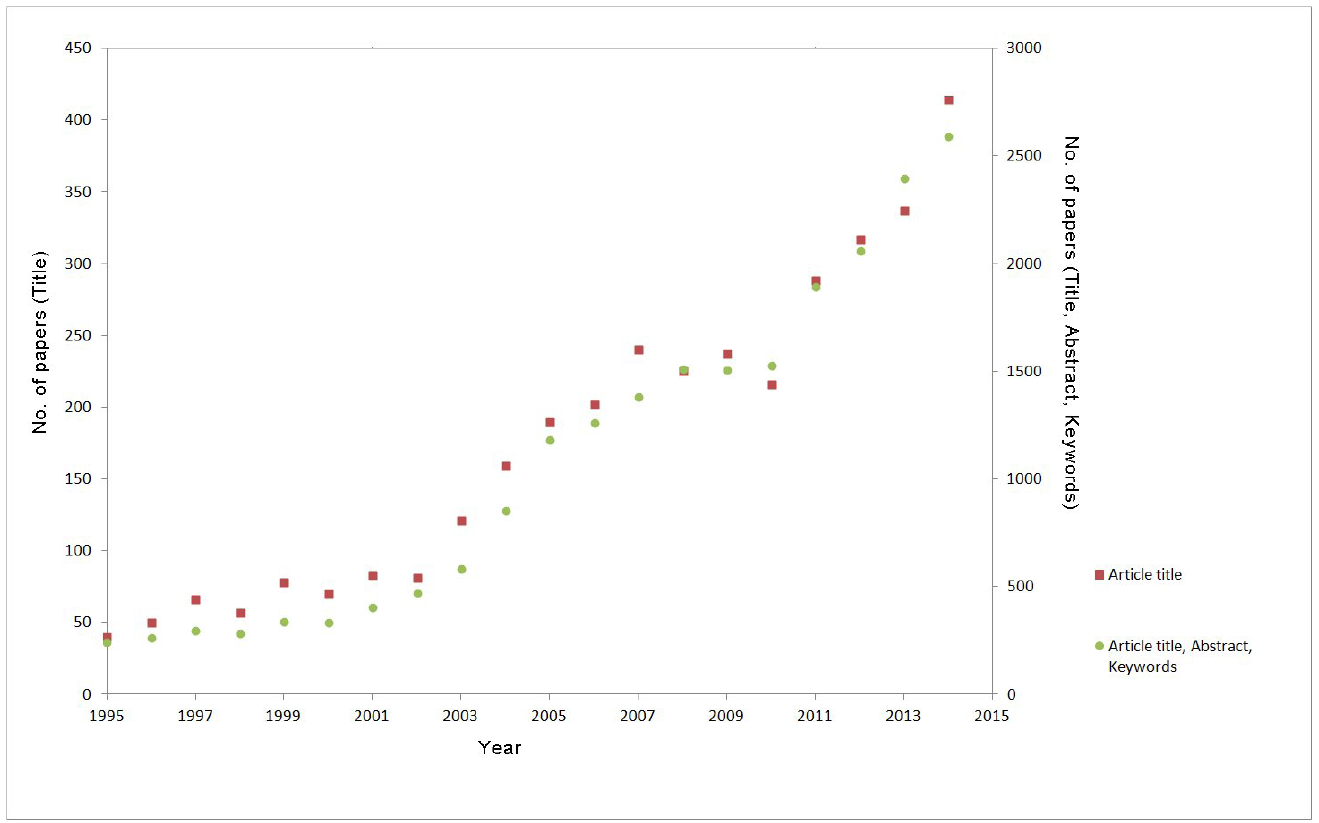
Fig.1. Number of published articles in scientific journals about Traditional Chinese medicine according to the Scopus database of peer- reviewed literature – searching for articles containing the term “traditional Chinese medicine” in article title (red squares) or in article title, abstract and keywords (green circles).
Saiga (Saiga tatarica) (Fig. 2) has populations of the subspecies Saiga tatarica tatarica in Kazakhstan, one of S. t. tatarica in Kalmykia, Russia, and two of S. t. mongolica in Mongolia. The Ustiurt population in Kazakhstan is relatively secure but is also under threat. The Mongolian subspecies show no evidence of decline, but the size of the population is relatively small (in 2015, the population was estimated to be 15,000 [9]). The data from the year 2001 [10] suggested that these three populations are under severe threat from poach- ing and have been declining at an increasing rate for years 1998-2000. The reason behind the decline in their population appears to be objective: wildfires, drought and as well as subjective: poaching for meat and horns.
For example, in May 2015, a catastrophic die-off of saiga population occurred in the Central Kazakhstan [9, 11]. According to an official statement by the Ministry of Agriculture (5th June), 62% of the population died in 2014 (134,252 individuals). Deaths were caused by the bacterium Pasteurella, however this pathogen only becomes fatal if the host immune system is weakened. The rapid collapse of this critically endangered antelope over a period of just 3 weeks is a terrible incidence.
Modern Chinese medicine is making efforts to reduce the demand for saiga horns. However “conventional” Chinese medicine has an even greater impact on the consumption of saiga horn. More than 30 TCM prescriptions or patent medicines contain horn of the saiga. In Newly Edited Chinese Traditional Patent Medicines published in 2002, Saiga horn
is depicted as the main component of 18 types of medicines for detoxification, treatment of cold and lung disease [12]. It is prescribed by the Chinese physicians for fever, dizziness, blurred vision, headache, and convulsion [13]. The damping, antipyretic, fever-lowering effects of the Saiga and other animal horns are known for centuries. Uncovering the identity of any biologically active compound(s) in the horns of these species could eventually lead to cheaper, more effective, synthetic alternatives and save these animals from extinction.
CHEMICAL COMPOSITION OF HORNS AND ANT- LERS
A plethora of methods have been described in connection to the analysis of TCM and are available in the published literature. Among all these modern methods therein, a com- bination of separation methods (LC) and mass spectrometry are frequently described nowadays (e.g. [14] [15]). Besides these research papers, numerous reviews (ten per year) also have been published on various aspects of TCM with respect to different diseases etc. including pharmacokinetics (e.g. [16]).
The chemical composition of horns includes microele- ments such as calcium, aluminium, chromium, copper, iron, manganese and zinc, the latter being the most important due to the belief that it could slow–down the aging process [17- 19]. All horns are composed of proteins and therefore, we can find a greater amount of amino acids in the hydrolysate of horns, the mass fraction of which varies between 30% to 80%, depending on the origin [20]. As mentioned earlier, the major component of any type of horn is keratin, but free amino acids, peptides, lipids, and nucleic acid residues are also present in the horns.

Fig.2. Wild male Saiga antelope (Saiga tatarica) on the morning steppe. Federal nature reserve Mekletinskii, Kalmykia, Russia. Copyright Victortyakht | Dreamstime.com http://www.dreamstime.com/victortyakht_info
14 nucleosides and nucleobases were determined in ani- mal horns, 12 of which have been quantified in saiga [21]. Hierarchical clustering analysis (HCA) and principal com- ponent analysis (PCA) divided the horns/animals into two groups (A and B), which is in agreement with taxonomy (25 horns were used from 7 species: Rhinoceros unicornis, Rhi- noceros bicornis, Pantholops hodgsoni. Saiga tatarica, Bos grunniens, Capra hircus, Bubalus bubalus). In the group A were rhinoceros horns, yak horns and water buffalo horns while tibetan antelope horns, saiga antelope horns and do- mestic ram, bulls cattle horns were in group B. These results suggest that it might be feasible to use water buffalo horns as a substitute for rhinoceros horns, and rams, bulls cattle horns as a substitute for saiga horns. This method could possibly provide a quick and effective search for substitutes from rare horns.
Another study [22] used three-stage infrared spectros- copy (Fourier transform infrared spectroscopy integrated with second derivative infrared spectroscopy and two- dimensional correlation infrared spectroscopy) for analysing the organic and inorganic compositions of three different horns (Cornu Antelopis, Cornu Bubali and Pulvis Cornus Bubali Concentratus). This method may also be considered as a candidate for rapid, non-destructive and effective analy- sis of traditional Chinese medicines and the identification of its constituents.
In search for an alternative, an aqueous extract of Cornu Bubali (water buffalo) horn was examined and some antipy- retic and antioxidant properties were described [23]. The same group of researchers identified three peptides with an- tioxidant properties, which were: Gln-Tyr-Asp-Gln-Gly-Val (WBH-1, 708 Da), Tyr-Glu-Asp-Cys-Thr-Asp-Cys-Gly-Asn (WBH-2, 1018 Da) and Ala-Ala-Asp-Asn-Ala-Asn-Glu-Leu- Phe-Pro-Pro-Asn (WBH-3, 1271 Da) [24]. The third peptide (designated HP) was determined in the horn of saiga at a level of 87.7 mg/kg, which is lower as compared to the Asi- atic rhino (Rhinoceri asiatici) (120.6 mg/kg) or African rhino (Rhinoceri africani) (138.8 mg/kg), but higher than the yaks (around 20 mg/kg) or the water buffalo (17.3 mg/kg) [25].
A comparative proteomic analysis, using two- dimensional electrophoresis, of the extracts prepared from the horns of rhinoceros, water buffalo, and yaks could iden- tify 14 common protein spots. A linear regression analysis established the correlation between pharmacological effica- cies and the identity of five active components, of which two protein spots were identified as Zinc finger CCCH-type con- taining 12D (CAX11887) and peptide methionine sulfoxide reductase (YP_001256455) [26] respectively, by using ma- trix-assisted laser desorption ionization time-of-flight mass spectrometry.
Proteomic analysis (high-performance liquid chromatog- raphy with mass-spectrometric -MS and MS/MS and poly- acrylamide gel electrophoresis) of the horns of saiga detected a high amount of keratin as expected, but additionally colla- gen type I was also found. Because the proteome of saiga is not yet described, the observed proteins were categorized according to the previously described proteomes of sheep and cattle – which identified them as keratin type I microfi- brillar (from sheep), keratin type II microfibrillar (from sheep), collagen type I (α(1)) (from bovine) and collagen
type I (α(2)) (from bovine), respectively. However, it is also important to mention that free amino acids were also present in these horns [27] and the free and total amino acids present in the horns are described in Table 1.
Chinese researchers performed an analysis of the amino acid hydrolysates of the horns from various animals (see Table 2) [28]. This work can be used as a reference and compared with the amino acid analysis (trimethylsilyl de- rivatives of amino acids) of 50% alcohol extracts of the horns of saiga and sheep by GC-MS [29]. In the latter work, the authors published a comparative data on the composition of the extracts of saiga and ram horns, and ram horn hydro- lysate extract, including the amino acids. The analysis clearly established the similarities between the amino acid composition of the saiga and the ram horn extracts and re- vealed the presence of secondary metabolites in the extracts, which could be divided into several groups: amino acids, hydroxy acids, the remnants of nucleic acids, steroids and fatty acids. The most important results regarding the differ- ences between the composition of saiga and the ram horn extracts are summarized in Table 3.
To compare the similarities between the compositions of the horn extracts, the formulations of sheep horns were com- pared using size exclusion chromatography in a Sephadex G25 column (using 0.1 mol/L acetic acid as the mobile phase). It showed that the extracts of Saiga and the ram horns have the same chromatographic profile which include seven peaks. There are only some minor differences in the proportions of individual components; for example, the ram horns contain more components with low mass and high retention times [20] as compared to the saiga horn.
The similarity between the amino acid and peptide com- position of the extracts was confirmed by comparing their physiological activity, such as anti-inflammatory response and decongestant properties, when extracts of saiga horns and sheep were compared. The antiulcer activity of 6 sub- stances extracted from the horns of saiga and sheep, respec- tively, was studied on the model of ethanol erosive ulcerous damage of the gastric mucosa of rats (Table 4). In these stud- ies [30, 31], fractions were dissolved in the physiological solution immediately before their administration, so that the volume was 1 ml per 200 g of animal weight when the dose was 2 or 10 mg/kg. The studied substances were adminis- tered intraperitoneally and intragastrically 1 hour before the administration of absolute ethanol (1 ml per 200 g weight, intragastrically through a tube). The sizes of the ulcerative lesions of the rat gastric mucosa were measured using a ruler under the binocular magnifying glass with a 10x magnifica- tion. To quantify the extent of the damage, an erosive index, expressed as mm/animal, was also determined [32]. The physiological activities elicited by the extracts of the horns of saiga and the sheep were similar (Table 4). From the re- sults, the authors concluded that the anti-ulcer activity is absent in the acetate extract. When the activity was com- pared between the extract of horns collected from saiga be- fore, during and after their rutting period, it became obvious that the protective activity was significantly lowered in the fraction of animals during the period of estrus (30% less) when the corresponding activity is important during and after the rut and it does not significantly differ (-71.5% and -66.3%). The most pronounced anti-ulcer activity demon- strated was fraction 7 (-62.4% – -79.9%, depending on the dose).
Table 1. Amino acid composition of horns.
|
Amino Acids |
Content, % | |||||||
|
Guan Horn [38] |
Buffalo Horn [38] |
Antlers of Young Sibe- rian Stags [20] |
Pantocrine [20] |
Horn of Saiga [19] | “Saitarin” Extract [19] | |||
|
Hydrolysate |
Free Amino Acids |
Hydrolysate |
Free Amino Acids | |||||
| Tryptophan | – | 0.57 | 0 | |||||
| Lysine | 13.39** | 3.35** | 6.3* | 3.76 | 9.4 | 13.4 | 4.3 | – |
| Histidine | 1.17 | 0.62 | 8.1 | 0.29 | 2.8 | traces | 0.6 | – |
| Arginin | 3.83 | 7.72 | 1.8 | 0.29 | 13.8 | 1.09 | 5.73 | 4.49 |
| Aspartic acid | 5.7 | 5.43 | 8.5 | 1.69 | 0 | – | 11.46 | 2.7 |
| Threonine | 4.75 | 3.65 | 3.8 | 1.41 | 9.5 | – | 5.73 | 2.24 |
| Serine | 9.68 | 5.61 | – | 7.42 | 9.4 | 12.2 | 8.6 | 8.98 |
| Glutamic acid | 13.15 | 12.61 | 4.6 | 4.90 | 2.2 | 3.66 | 17.19 | 4.49 |
| Proline | 2.37 | 4.42 | 11.5 | 4.29 | 4.8 | 7.3 | 7.45 | 6.28 |
| Glycine | 4.3 | 2.73 | 10.2 | 11.12 | 7 | 9.15 | 7.16 | 20.65 |
| Alanine | 3.67 | 3.24 | 15.5 | 18.11 | 6.5 | 0.73 | – | 8.98 |
| Valine | 3.06 | 3.84 | 9.9 | 16.68 | 7.9 | 7.3 | 5.73 | 14.4 |
| Isoleucine | 2.16 | 2.66 | 4.2 | 0.57 | 5.3 | 6.1 | 3.72 | 8.98 |
| Leucine | 5.95 | 6.66 | 1.6 | 6.31 | 12.7 | 9.76 | 11.46 | 17.95 |
| Tyrosine | 2.49 | 2.95 | 7.4 | 1.14 | 4.8 | 18.3 | 6.59 | – |
| Phenylalanine | 1.20 | 2.31 | 1.4 | 3.15 | 4.7 | 10.9 | 4.3 | – |
| Cysteine | 4.2 | 0.80 | ||||||
| Taurine | – | 0.57 | ||||||
| Urea | 1.42 | |||||||
| Methionine Sulphoxide |
0.57 |
|||||||
| Sarcosine | 0.57 | |||||||
| Glucosamine | 0.97 | |||||||
| 69.25 | 73.08 | 101.1 | 100.2 | 100.8 | 99.89 | 100.02 | 100.14 | |
*- After hydrolysis of 100 g of powdered antler with hydrochloric acid
** – 100 g of horns
A Saiga-horn extract called ‘saitarin’ was reported to have antipyretic and anti-ulcerative properties [17, 18, 30, 31, 33-35]. Moreover, the 7th fraction, which was also the last fraction obtained from the Sephadex G-25 columns (separation of compounds according to molecular weight), was reported to have the greatest effect [33]. The presence of antiulcer effect was reported in an extract from the horn of saiga after rutting [31]. Rutting represents a period of sig- nificantly high level of stress, both physical and emotional (psychological). For example, male saiga can capture group from 2-3 to 20-25, and in rare cases, 50 females graze these “family herd,” or harems, for 15-20 days of December and set out constant clashes with rivals. All this time, they almost do not feed but often eat snow. Weakened by the excessive fasting, males more often than females become the prey of wolves and in the case of unfavorable wintering in mass, die from exhaustion.
Antipyretic effects have been reported not only from the horns of rhinoceros, but also in the horns of water buffalo, cattle and saiga. All of these horns have an antipyretic effect at a high dose, as indicated by experimental studies on rats (5 g/ml/rat, which is equal to over 100 times the normal hu- man oral dose), with the exception of only rhino and saiga , for which a positive effect has been observed even at a lower dose (1 g/ml). [3]. However, the same authors demonstrated that a comparable antipyretic effect can be achieved by both rhinoceros as well as buffalo horns in Qingying Decoction, a classic compound prescription composed of rhinoceros horn and eight different herbs. [4]. It thus appears that the antipy- retic effect is not strictly dependent on the origin of the horn, and rather, might be connected to the herbal substances pre- scribed and used in the preparation of Chinese medicine. We should also stress here that some authors [3, 4, 23] have even demonstrated that an antipyretic effect is characteristic to all animal horns (e.g. antelope, cattle, buffalo, rhino, yak).
Horns as well as the tendons, according to Tibetan medi- cine may contain substances that not only affect the tissue itself, but also the state of the whole organism [36]. It should be noted that the peptides detected in the horn extracts can exhibit regulatory functions in the body and thus provide the therapeutic effect. Each individual has a unique tissue that constitutes the body of an animal and, contains substances that allow them to regulate their “normal” function (bioregu- lation of tissue) [37]. Many peptides isolated from different tissues exhibit a physiological activity. Moreover, it may be tissue-specific action, i.e. specific to each organ but it can also have a more general regulatory function. The study of tissue-specific mechanisms of action of peptides is an actual problem of modern molecular biology, physiology and medicine. Most often, tissue-specific action of peptides (as well as other substances or effects) implies mechanisms hav- ing their impact on various organs or tissues. It is most likely that the main function of tissue-specific peptide would be to control or regulate the biochemical processes of the cells corresponding to that specific tissue. It is noteworthy that an active principle, in this case is not caused by the individual components but by a variety of peptides with mixed activity.
In accordance with the modern concept of peptide bioregulation, it is thought that endogenous peptides (pep- tides produced in the cell itself) participate in maintaining the structural and functional homeostasis of the same popula- tion of cells which contain and produce these factors. There- fore, it is of fundamental importance [37] to study the struc- tural-functional features of these substances in order to de- velop proposals for the mechanisms of action of tissue- specific peptides. Thus, a comprehensible and necessary starting point would be to study the structure of the active substances and test their physiological activities.
Table 2. Amino acid content in the hydrolysate of horn samples (%) [28].
|
Amino acid |
Tibetan antelope |
Saiga |
White rhinoceros | Indian rhinoceros |
Buffalo horn |
Black yak |
Color yak |
White yak |
| Aspartate | 8.58 | 8.78 | 7.70 | 8.51 | 6.95 | 7.52 | 7.95 | 7.70 |
| Threonine | 4.99 | 4.80 | 4.86 | 3.95 | 4.83 | 5.05 | 5.69 | 5.29 |
| Serin | 6.52 | 5.99 | 6.67 | 5.04 | 6.90 | 6.02 | 7.21 | 6.75 |
| Glutamate | 16.59 | 16.54 | 15.02 | 15.31 | 12.59 | 14.10 | 14.99 | 14.64 |
| Proline | 4.14 | 3.69 | 4.64 | 3.46 | 5.35 | 4.79 | 5.20 | 4.78 |
| Glycine | 4.11 | 4.05 | 4.33 | 4.95 | 6.76 | 5.30 | 4.66 | 4.50 |
| Alanine | 4.79 | 4.65 | 4.50 | 4.88 | 3.65 | 3.88 | 4.19 | 4.05 |
| Cysteine | 5.79 | 5.70 | 6.94 | 3.66 | 8.35 | 7.95 | 9.06 | 8.13 |
| Valine | 5.96 | 5.77 | 5.60 | 5.63 | 4.67 | 5.77 | 6.02 | 5.76 |
| Methionine | 1.37 | 1.36 | 1.09 | 1.40 | 0.41 | 1.39 | 1.49 | 1.33 |
| Isoleucine | 3.79 | 3.90 | 3.54 | 3.95 | 3.29 | 3.52 | 3.84 | 3.77 |
| Leucine | 10.19 | 10.21 | 9.19 | 9.99 | 8.50 | 8.58 | 8.97 | 8.78 |
| Tyrosine | 4.12 | 4.01 | 5.12 | 5.16 | 8.05 | 6.06 | 5.02 | 4.51 |
| Phenylalanine | 2.46 | 2.59 | 2.42 | 3.02 | 3.00 | 2.35 | 2.33 | 2.34 |
| Lysine | 4.51 | 4.61 | 3.46 | 4.39 | 3.46 | 3.65 | 3.95 | 3.75 |
| Histidine | 1.06 | 1.08 | 1.00 | 1.39 | 1.01 | 0.84 | 0.90 | 0.89 |
| Tryptophan | 0.66 | 0.64 | 0.75 | 0.82 | 0.74 | 0.56 | 0.66 | 0.65 |
| Arginine | 10.55 | 10.23 | 11.25 | 10.18 | 10.00 | 10.29 | 11.28 | 11.07 |
Table 3. Main components of extracts of saiga and ram, and hydrolysate of extract of saiga by GC-MS after derivatization with BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) [29].
| Extract | |||
| Extract Components of Saiga*,% | Extract Components of Ram*,% | Hydrolysate Extract of Saiga* | |
| Lactic acid | 9.9 | 16.34 | 1.48 |
| Acetic acid | 1.64 | – | 0.63 |
| Alanine | 2.82 | 5.95 | 4.72 |
| Glycine | 1.99 | 3.28 | 14.45 |
| Butanoic-3-oxy acid | 0.16 | 0.25 | 0.03 |
| Valine | 3.38 | 3.28 | 2.7 |
| Urea | 4.6 | 2.89 | 0.08 |
| Leucine | 2.63 | 3.74 | 5.19 |
| Proline | 1.92 | 2.89 | 9.25 |
| Pyrimidine-2,4-bisoxy | 1.11 | 1.42 | 0.55 |
| Serine | 3.37 | 6.11 | 11.77 |
| Threonine | 1.93 | 2.68 | 6.69 |
| Aspartic acid | – | – | 7.15 |
(Table 3) contd….
| Extract | |||
| Extract Components of Saiga*,% | Extract Components of Ram*,% | Hydrolysate Extract of Saiga* | |
| Proline-4-oxo | – | – | 0.81 |
| Proline-4-oxy | 1.11 | 0.48 | – |
| Glutamine | 1.3 | 0.9 | – |
| Cysteine | – | – | 0.39 |
| Ornithine | – | – | 0.25 |
| Glutamic acid | – | – | 6.36 |
| phenylalanine | 1.77 | 1.85 | 3.04 |
| Ornithine | 1.06 | 0.06 | 0.3 |
| Lysine | 0.64 | 0.34 | 2.03 |
| Tyrosine | 2.25 | 2.28 | 5.6 |
| myo-Inositol | 1.52 | 2.06 | 0.6 |
| 6-oxy-2-aminopurin | 1.64 | 1.58 | – |
| Hexadecanoic acid | 1.3 | 1.08 | – |
* – the probability of coincidence with the standard 80%
Table 4. Antiulcer activity of extracts of horns on the model of ethanol ulcers [29].
| Substance | Dose, mg/kg | Number of animals | Erosive index, mm/animal | Inhibition of damage, % |
|
Saiga – water extract (before rut)1 |
10 – inside of the stomach | 3 | 56.7 ±5.26 | 0 |
| 2 – inside of the stomach | 3 | 11.7 ± 3.29 | -76.6 | |
| Saiga after rutting2 | 10 – inside peritoneal | 5 | 18.2 ± 4.87 | -66.3 |
| Ram – coarse breed of ram 3 | 10 – inside peritoneal | 6 | 42.3 ± 6.21 | -49.9 |
| Ram – Merino fine-fleeced breed4 | 10 – inside peritoneal | 6 | 29.3 ± 2.61 | -62.3 |
| Ram – Merino fine-fleeced breed 7th fr.5 | 10 – inside peritoneal | 1 | 17 | -79.9 |
|
Saiga before rutting 7th fr.6 |
2 – inside peritoneal | 6 | 17.0 ± 4.5 | -79.9 |
| 0.2 – inside peritoneal | 6 | 31.8 ± 4.2 | -62.4 |
1Water extract from the top of horns obtained from males up to rutting
2Extract from the top of horn obtained from males after rutting (50% ethanol extract) (average age of male was 1.7 years)
3Extract of horn of sheep (ram), rough wool breed
4Extract of horn of sheep, fine wool breed (Merino) (Ram – Merino fine-fleeced breed)
5Extract of horn of sheep, fine wool breed (Merino) (Ram – Merino fine-fleeced breed). 7th fraction after separation on Sephadex G25
6Extract from the top of horn obtained from males before rutting (average age of male was 1.7 years). 7th fraction after separation on the Sephadex G25
CONCLUSION
Saiga horns are popular in traditional Chinese medicine. The main beneficial effects of the horn, which could be de- scribed in scientific terms, are mostly antipyretic and antiul- cerative. Despite some indication of its efficacy, no specific compound from the horn has yet been isolated or scientifi- cally described. If someone believes in the positive influence of animal horns on human health, we strongly recommend the use of a horn from another, less critically endangered species as saiga (or rhinoceros) animal: water buffalo, bulls cattle or rams. However, the research of TCM can obtain some new relevant results. Beneficial effects are not assumed but these should be evident from thousands years of use. The matter is that we need to identify the effective component(s) and understand its mechanisms of action.
Source: Research Gate