1. Introduction
Bear bile is a valuable and significant medicinal material in the practice of traditional Chinese medicine (TCM), with a history of use in China spanning over 13 centuries. In TCM, bear bile is believed to possess properties that can ‘‘clear heat” and ‘‘elimi- nate liver fire,” which have been traditionally associated with dis- eases such as hepatobiliary disorders, as well as eye and throat infections. Bear bile is primarily composed of bile acids (BAs), including ursodeoxycholic acid (UDCA), chenodeoxycholic acid (CDCA), and their taurine conjugates. Recent research has revealed that BAs play a much more significant role than merely acting as ‘‘digestive surfactants”. In fact, they have been identified as signaling molecules capable of interacting with multiple receptors such as farnesoid X receptor (FXR), G-protein-coupled bile acid receptor, Gpbar1 (TGR5), vita- min D receptor (VDR) , and sphingosine-1-phosphate receptor-2 (S1PR2), thus serving as regulators of host metabo- lism and inflammation, and mediating communication between the gut and liver. BAs and their derivatives have demonstrated promising therapeutic potential in treating a range of diseases, such as liver diseases, metabolic disorders, inflamma- tory bowel diseases, and neurodegenerative diseases. Moreover, UDCA may play a significant role in managing coronavirus disease 2019 (COVID-19) by reducing FXR signaling, down-regulating angiotensin-converting enzyme 2 (ACE2) expres- sion, and decreasing susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectio.
In TCM practice, bear bile has been incorporated into herbal remedies for treating diverse ailments, including diabetes, nephri- tis, chin cough, hemorrhoids, and chronic hepatitis B-induced jaun- dice. At present, 40 tons of bear bile are annually required by 87 Chinese traditional patent medicines involving 267 pharmaceu- tical enterprises. Historically, wild black bears were hunted for their bile until the population of black bears drastically declined. Subsequently, Ursus thibetanus (Asiatic black bear) and Ursus arctos (brown bear), the two primary species of bears in China, were listed as Grade II protected in China, and cross-border trade was prohibited by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). In the 1980s, China banned bear hunting, leading to the inception of bear bile farming (i.e., drained bear bile (DBB)), which employs the ‘‘free-dripping fistula” technique to extract bile from living bears through a cathe- ter or pipe inserted into their gallbladder. Bear bile farming is now the sole legal source of bear bile available on the Chinese market. Although extracting bile from live bears was initially consid- ered more ethical than killing them, bear bile farming is currently under scrutiny by animal welfare advocates and the public world- wide for its persistent cruelty and adverse impacts on the physical and mental health of bears.
There is a pressing need to create an alternative for bear bile in order to reconcile medical demands and bear protection. Research has explored the use of bile from other livestock or poultry, single BAs such as UDCA, and TCM herbs as substitutes for bear bile. However, due to the complex chemical composition and diverse pharmacological effects of bear bile, there is still insuffi- cient experimental evidence to support their use. Ideally, alternative options should closely match the chemical and phar- macological properties of bear bile, while offering more stable and consistent quality and enhanced safety. The creation of artificial bear bile (ABB) poses several obstacles. First, modern medical terminology does not correspond with the traditional indi- cations of bear bile, which presents a significant challenge in eluci- dating the scientific basis of bear bile’s medical uses. Second, even though an increasing number of studies have been published on bear bile and its components, the relationship between its function and chemical composition remains unclear. Finally, achieving a rational composition ratio for substitutes is challenging due to the chemical diversity and pharmacological versatility of bear bile constituents, coupled with the synergistic effects between them.
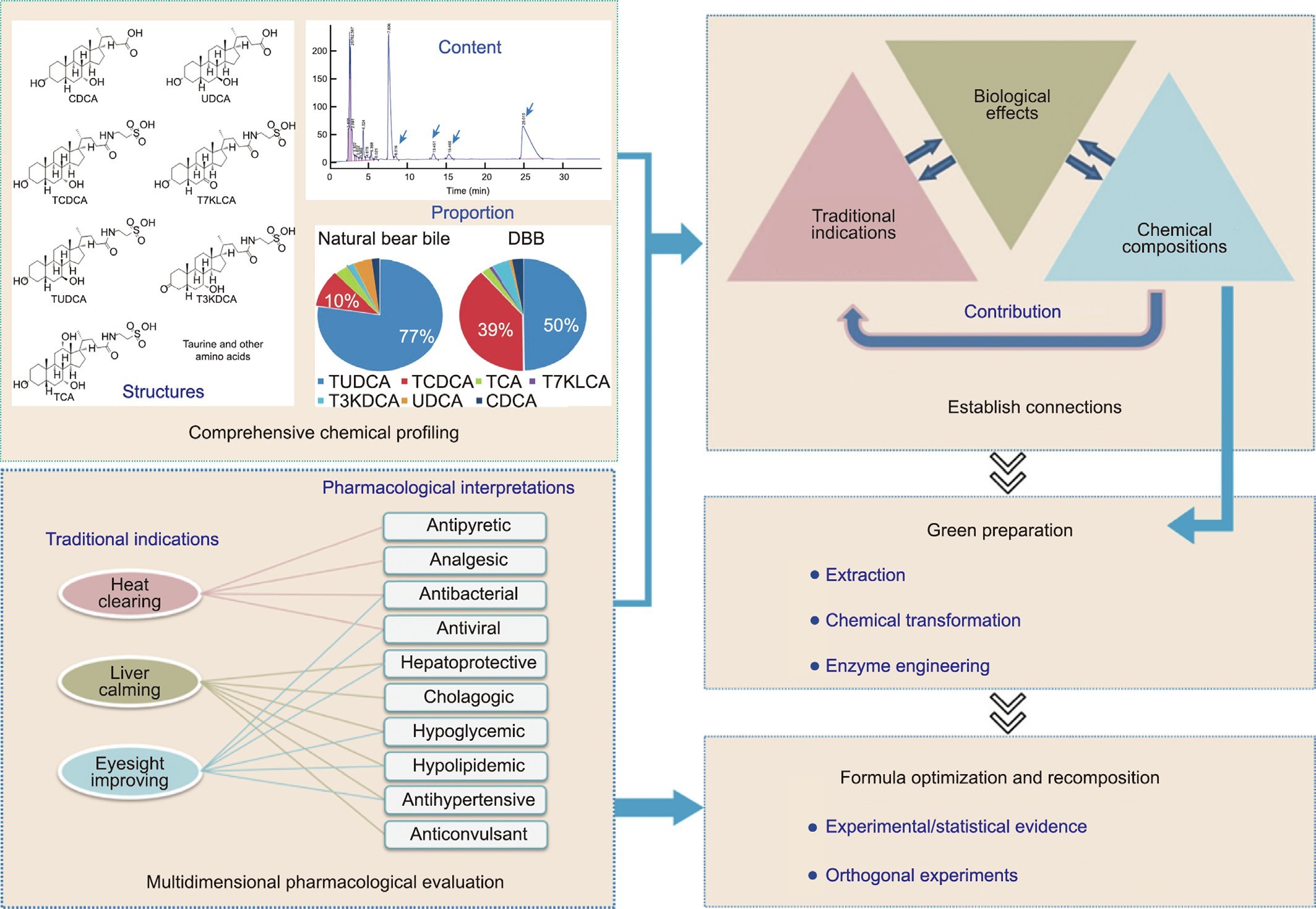
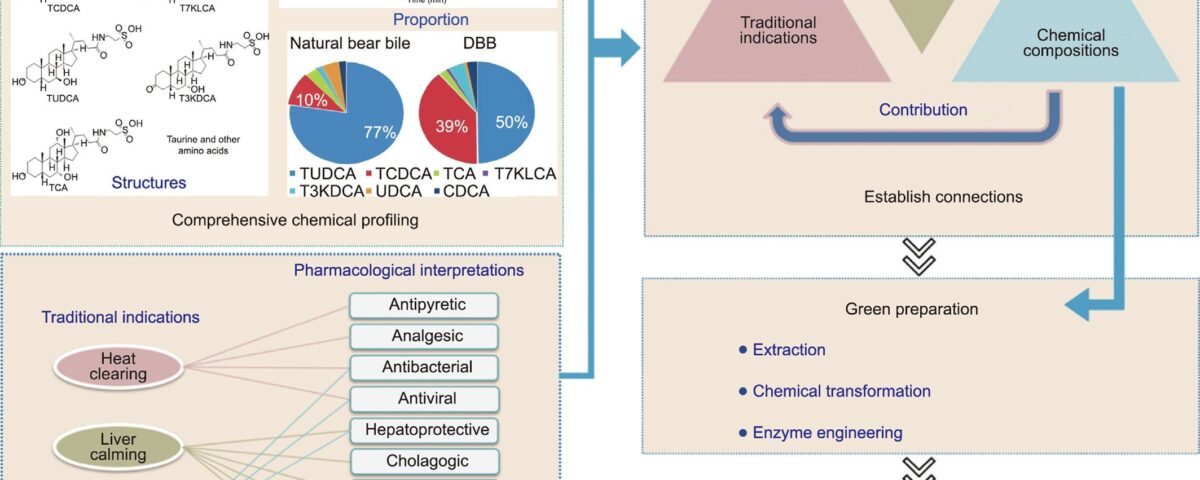
Fig. 1. The new proposed strategy to create ABB. TCDCA: taurochenodeoxycholate; T7KLCA: tauro-7-ketolithocholic acid; TUDCA: tauroursodeoxycholate; T3KDCA: tauro- 7a-hydroxy-3-oxo-5b-cholanoate; TCA: taurocholic acid.
To tackle these challenges, we propose a novel approach for developing ABB (outlined in Fig. 1), which is based on a systematic investigation of the scientific foundation underlying the traditional effectiveness of bear bile. The proposed strategy consists of several key steps:
(1) A comprehensive characterization of the structure, type, content, and proportion of chemical components found in bear bile;
(2) Development of a multidimensional pharmacological model system that can elucidate the traditional indications of bear bile and reflect TCM’s clinical experience;
(3) Establishment of the connections among traditional indica- tions, biological effects, and chemical compositions;
(4) Determination of the contributions of various components’ efficacy;
(5) Integration of technologies from chemistry, enzyme engi- neering, and other related fields to achieve the green preparation of the potent constituents of bear bile;
(6) Optimization and recomposition of ABB formulations based on experimental and statistical evidence.
Herein, we present a novel approach for the green production of an ABB that matches natural bear bile’s chemical and pharmaco- logical properties. By leveraging a new research strategy and incor- porating multidisciplinary technologies, we have succeeded in developing an alternative to bear bile farming that does not depend on any wild or endangered resources. Our solution offers several advantages, including environmental sustainability and consistent, reliable quality control.
2. Materials and methods
2.1. Materials and reagents
Zhongshan Belling Biotechnology Co., Ltd. (China) provided 3a- hydroxysteroid dehydrogenase (3a-HSDH), lactate dehydrogenase (LDH), 7a-hydroxysteroid dehydrogenase (7a-HSDH), 7b- hydroxysteroid dehydrogenase (7b-HSDH), glutamate dehydroge- nase (GDH), taurine, and bile pastes from chicken, duck, goose, and cattle. Nicotinamide adenine dinucleotides (NAD) and nicoti-namide adenine dinucleotide phosphate (NADP) were procured from Bontac Bio- engineering (Shenzhen) Co., Ltd. (China), while D101 macroporous adsorption resin was obtained from Cangzhou Bonchem Co., Ltd. (China). ICR mice, Sprague–Dawley (SD) rats, and C57 mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China); and Wistar Kyoto (WKY) rats, spontaneously hypertensive (SHR) rats, KKAy mice, and Kunming mice were obtained from Beijing HFK Bioscience Co., Ltd. (China). The virus strain and cells were obtained from American Type Culture Collection (ATCC). Influenza infection experiments in mice were conducted in the animal biosafety level 2 (ABSL-2) labora- tory, following the International Committee on Animal Ethics stan- dard operating procedures, and the Animal Ethics Committee guidelines of the Institute of Pharmaceutical Biotechnology and Institute of Materia Medica of the Chinese Academy of Medical Sciences (CAMS). The China National Traditional Chinese Medicine Corporation provided DBB powder samples, while intact natural bear bile samples were donated by Mr. Xiao Yun Wang and identi- fied by Prof. Lin Ma of the Institute of Materia Medica, CAMS, through methods including visual examination, physical characteristics, and high-performance liquid chromatography (HPLC) analysis. The natural bear bile samples used in this project were approved by the Beijing Landscape and Greening Bureau.
Aspirin, lipopolysaccharides (LPS), concanavalin A (Con-A) pro- tein, pentylenetetrazole (PTZ), a-naphthalene isothiocyanate (ANIT), 2,3,5-triphenyltetrazolium chloride, and gabapentin were procured from Sigma-Aldrich (Germany). Nifedipine was supplied by Yangtze River Pharmaceutical (Group) Co., Ltd. (China), while Inno-Chem (China) provided carbamazepine (CBZ) and Beijing Union Pharmaceutical Factory (China) supplied bicyclol. Diammo- nium glycyrrhizinate (GRZ) injection was purchased from Chia-Tai Tianqing Pharmaceutical Co., Ltd. (China). Ribavirin was supplied by Xinxiang Pharmaceutical Co., Ltd. (China), and Tamiflu was purchased from Shanghai Roche Pharmaceutical Co., Ltd. (China). Berberine hydrochloride, levofloxacin, and oseltamivir phosphate were obtained from the National Institute for Food and Drug Control, China. Alanine aminotransferase (ALT), aspartate amino- transferase (AST), glucose, total cholesterol (CHO), total bilirubin, and triglyceride (TG) assay kits were purchased from BioSino Bio-Technology & Science Inc. (China).
2.2. Efficacy evaluation models
2.2.1. Rat fever model induced by yeast
Rats with body temperatures in the range of 36.2–37.2 °C were randomly assigned to groups of ten. The positive control drug aspirin and the test sample were orally administered at a dose vol- ume of 4 mL∙kg—1, followed by a subcutaneous injection of 20% yeast suspension (10 mL∙kg—1). The control group received an injection of saline. The rats’ temperatures were monitored after 2 h and again at 6 and 8 h after the oral administration of aspirin or samples.
2.2.2. Rat fever model induced by LPS
After oral administration of aspirin or samples, rats were injected intraperitoneally with LPS suspension (20 lg∙kg—1, 2 mL∙kg—1), while the normal control group was injected with sal- ine. After 2 h of temperature monitoring, the rats were orally administered aspirin and samples again, and their temperatures
were measured at 6 and 8 h after injection, for a total of 8 h of monitoring.
2.2.3. Hot plate test
ICR mice were screened for pain threshold on the first day (via a hot plate temperature set at (55 ± 0.2) °C), and those with thresh- olds between 10–30 s were selected, randomly divided into groups of ten, weighed, and labeled. Aspirin, gabapentin, and samples were orally administered (10 mL∙kg—1) on the first day, followed by the same administration the next day, with a pain threshold measurement taken 1 h later.
2.2.4. Acetic acid writhing test
Mice were given aspirin and samples orally 1 h before receiving an intraperitoneal (ip) injection of 0.6% acetic acid solution to observe latency and the number of writhing events within 15 min.
2.2.5. In vivo anti-influenza virus effects
After 1–2 d of adaptive feeding, the mice were randomly grouped, with ten mice in each group. The mice were infected with influenza A virus (H1N1) strain A/FM/1/47 via nasal drops while under anesthesia, at 0.035 mL per mouse (35TCID50) once daily for 7 d, with deaths within 14 d recorded. Survival rates, average life expectancy, death protection rates, and prolonged life expec- tancy were calculated for each group. Two dosing regimens were implemented in the experiment, with the first dose being adminis- tered either 2 or 24 h after infection. Administration was carried out via oral gavage for a total of 7 d.
2.2.6. Anticonvulsant effects
Mice were observed for 30 min after receiving an ip injection of PTZ, with seizure latency and scores being recorded. PTZ injections were given every other days until all mice had grade-3 and higher seizures; then, PTZ injections were given for three consecutive days. One month later, an equal dose of PTZ was given to stimulate seizures, and mice producing grade-3 and higher seizures were used for evaluation, being scored up to a maximum of 5 based on seizure severity.
2.2.7. Protection of liver injury induced by CCl4
Each group of ICR female mice (20–25 g) received three doses every 12 h. One hour after the last administration, except for the normal control group, 0.2% CCl4 was injected intraperitoneally. After the mice fasted overnight for 16 h, blood was drawn and cen- trifuged at 3500 r∙min—1 for 10 min. The pellet was discarded, and the supernatant was diluted with physiological saline (ten times) to detect ALT and AST using an automated biochemical analyzer.
2.2.8. Protection of immune liver injury induced by Con-A
For ICR mice, three doses were administered every 12 h, with the normal control and model groups receiving an equal amount of water. One hour after the last administration, a tail vein injec- tion of 20 mg∙kg—1 (10 mL∙kg—1) Con-A was given. After the mice fasted overnight for 16 h, blood ALT and AST levels were detected using an automated biochemical analyzer.
2.2.9. Protection of ANIT-induced cholestatic jaundice
ICR mice received drug administration for 4 consecutive days, with ANIT (120 mg∙kg—1) being orally administered 2 h after the drug administration on day 2. After the mice fasted overnight for 16 h, blood ALT, AST, and total bilirubin (TBIL) levels were detected using an automated biochemical analyzer.
2.2.10. Hypoglycemic and hypolipidemic effects
The KKAy mice were fed a high-fat feed, while the C57 mice were provided with normal feed. The KKAy mice (15–17 weeks) underwent fasting (2 h) glucose and lipid measurements before the experiment. Mice were grouped based on glucose, lipid, and body weight levels to ensure a consistent mean and standard deviation in each group. The drug was administered for 4 consecutive days (0.1 mL per 10 g weight), with the final dose given by tube after 1 h of fasting, followed by measurement of the fasting glucose and lipid values after 1 h.
2.2.11. Antihypertensive effects
For the WKY and SHR rats, body weight, blood pressure, and heart rate were monitored until eight weeks of age. Nifedipine or samples were orally administered for 14 consecutive days (with distilled water given to the WKY control and SHR model groups). Body weight, blood pressure, and heart rate were monitored on days 4, 8, 14, and 21.
3. Results
3.1. Clarification of the scientific rationale for the medicinal use of bear bile
In order to develop alternatives to bear bile, it is crucial to clar- ify the scientific reasoning behind its medicinal use. Despite exten- sive research on the chemical composition and biological effects of bear bile, a comprehensive understanding is still lacking. To make further progress, a holistic approach that combines both chemical and pharmacological investigations is needed.
3.1.1. Systematic chemical profile
To obtain a comprehensive understanding of the composition and proportions of the substances present in bear bile, a systematic chemical profile was conducted on a large sample size. A total of 73 batches of DBB and three natural bear bile samples were analyzed. The results, displayed in Table 1, revealed the presence of seven BAs with content above 0.5% in drained or natural bear bile, includ- ing tauroursodeoxycholate (TUDCA), taurochenodeoxycholate (TCDCA), taurocholic acid (TCA), tauro-7-ketolithocholic acid (T7KLCA), tauro-7a-hydroxy-3-oxo-5b-cholanoate (T3KDCA), UDCA, and CDCA, which together accounted for 56.20%–82.70% of the dry weight of bear bile. Furthermore, cold-spray ionization mass spectrometry (CSI-MS) analysis confirmed that the conjugated BAs were present in the form of sodium salts.
It was notable that the mean TUDCA content in DBB was 33.10%, which was considerably lower than that in natural bear bile (59.90%). In addition, the ratio of TUDCA to TCDCA was around six times greater in natural bear bile (a mean of 8.3 for three sam- ples) compared with DBB (a mean of 1.3 for 69 samples)-a finding that is consistent with reports from various research groups. It is unclear whether the drainage procedures contribute to these discrepancies. Nevertheless, TUDCA levels are a crucial indi- cator of the quality of bear bile due to its multiple biological effects, which include modulatory effects on endoplasmic reticu- lum (ER) stress and oxidative stress, anti-apoptotic properties, and anti-inflammatory properties. On the other hand, TCDCA, a natural ligand for receptors such as FXR, is associated with both regulating glucolipid metabolism and negative conse- quences, such as diarrhea and increased transaminases. There- fore, variations in the composition of bear bile can undoubtedly impact its efficacy and safety.
In addition to BAs, bear bile contains amino acids, fatty acids, ash content, moisture content, and trace amounts of bilirubin. To compare the protein components in fresh bear bile juice with those in dried bear bile powder, polyacrylamide gel electrophoresis (PAGE) analyses were conducted. Unlike dried DBB or natural bear bile, fresh bear bile juice exhibited significant bands at 25, 60, 70, and 130 kD (Fig. S1 in Appendix A). Further studies revealed that proteins in the bile may break down within a week at ambient temperature during the drying process (Fig. S2 in Appendix A).
Table 1
Content of BAs in natural bear bile and DBB.
BA DBB (%) Natural bear bile (%)
| Average | Standard deviation | Range of content | Average | Standard deviation | Range of content | |||
| TUDCA-Na | 33.10 | 3.72 | 26.40–42.80 | 59.90 | 11.03 | 49.60–71.50 | ||
| TCDCA-Na | 25.90 | 2.99 | 19.00–32.50 | 8.07 | 2.72 | 5.59–11.00 | ||
| TCA-Na | 1.54 | 0.39 | 0.81–2.54 | 2.70 | 1.84 | 0.78–4.44 | ||
| T7KLCA-Na | 0.58 | 0.13 | 0.36–1.12 | 0.17 | 0.07 | 0.10–0.21 | ||
| T3KDCA-Na | 3.11 | 0.59 | 2.33–5.05 | 1.24 | 0.13 | 1.10–1.33 | ||
| UDCA | 0.51 | 0.30 | 0–1.68 | 3.80 | 3.21 | 0.54–6.95 | ||
| CDCA | 1.79 | 1.23 | 0.10–5.41 | 1.36 | 1.66 | 0–3.21 | ||
| Total BA | 66.40 | 4.58 | 56.20–82.70 | 77.20 | 2.27 | 75.30–79.70 |
An amino acid analysis showed that there are over 16 amino acids in bear bile, with taurine being the most abundant amino acid, accounting for 0.54% of the weight of dried bear bile powder.
A comprehensive qualitative and quantitative analysis was con- ducted to determine the mean values, content ranges, and degrees of variation of the components in drained and natural bear bile (Table 1). This analysis provided a reference and basis for the creation of ABB.
3.1.2. Bridging the gap between the traditional indications and pharmacological effects of bear bile
The discrepancy between traditional indications for bear bile and modern medical terminology can be attributed to the funda- mental differences between TCM and modern medicine. TCM prac- tices are rooted in a distinct theoretical system that utilizes unique diagnostic criteria and emphasizes holistic approaches to health maintenance. Their traditional indications usually stem from observations of phenotypic changes rather than specific disease diagnoses. Moreover, the complexity of the herbal formulas used in TCM, in which bear bile is primarily utilized in combination with other herbs, poses difficulties in isolating the specific contri- bution of each ingredient.
The establishment of connections between traditional indica- tions and pharmacological effects (as illustrated in Fig. 1) was pri- marily based on TCM theory, clinical application experience, and modern pharmacological research on bear bile. Given the lack of a strict one-to-one correspondence between traditional indications and modern pharmacological effects, it was important to empha- size the pivotal role of the clinical applications of bear bile in bridg- ing the gap. Clinical applications not only reflect the principles of TCM, but can also be interpreted from a modern pharmacological perspective.
‘‘Heat clearing” is a term used in TCM to describe therapeutic interventions aimed at treating symptoms associated with ‘‘exces- sive internal heat,” such as fever, dry mouth, sore throat, and con- stipation. Such symptoms are often correlated with infectious diseases and inflammatory disorders, among others.
The therapeutic intervention of ‘‘liver calming” in TCM addresses symptoms related to imbalances in the flow of ‘‘qi” energy within the liver meridian, which can give rise to irritability, mood swings, and digestive disorders. In clinical practice, the phar- macological effects of liver protection, cholestasis, hypoglycemia, hypotension, and anticonvulsant properties have been associated with this approach.
‘‘Eyesight improving” is a term used to describe the treatment of eye diseases and associated symptoms, such as redness, itching, dryness, and blurred vision. According to TCM theory, ‘‘nourishing the liver and kidney meridians” can play an essential role in pro- moting improved eye health, in addition to well-known antiviral and antibacterial therapies. As a result, clinical interventions aimed at liver protection, blood pressure reduction, and cholesterol and blood sugar regulation are often considered to be integral compo- nents of ‘‘eyesight improving” therapies to help improve overall patient outcomes.
A multidimensional pharmacological model system was estab- lished based on relationship mapping. To evaluate the applicability of the pharmacological model system in assessing the therapeutic effects of bear bile, nine batches of bile from three regions were tested. Detailed results can be found in Tables S1–S10 in Appendix
A. Based on an analysis of the experimental data, individual scores were assigned to each sample for its therapeutic effect in each model. Overall, the bear bile samples exhibited excellent therapeu- tic effects in the antipyretic, analgesic, hepatoprotective, chola- gogic, hypoglycemic, and hypolipidemic models. Therefore, our optimized multidimensional pharmacological assessment system can largely represent the traditional indications of bear bile and reflect the clinical experience of TCM, making it applicable for eval- uating the contribution of various components in bear bile and the efficacy of potential alternatives.
To better comprehend the contributions of various types of bear bile components to the overall efficacy, bear bile was separated into fractions such as total amino acids (TAAs), total taurine- conjugated bile acids (TTCBAs), and total free bile acids (TFBAs) (the isolation process is shown in Fig. S3 in Appendix A). These were then validated by testing their effects on the evaluation sys- tem (Tables S11–S13 in Appendix A). Each fraction was scored based on the significance of its effects (Table S14 in Appendix A). The results showed that TAAs had significant effects on the antipyretic models. TTCBAs exhibited notable effects on the hepa- toprotective, cholestatic, and lipid-lowering models, while TFBAs had certain effects on the antipyretic, hepatoprotective, hypo- glycemic, and lipid-lowering models. Therefore, TAAs, TTCBAs, and TFBAs all play important roles in bear bile, with none of the single components possessing the full spectrum of efficacy of the whole bear bile.
3.2. Creation of ABB
Among the many medicinal substances in bear bile, BAs func- tion as signaling molecules by binding and activating nuclear receptors such as FXR, pregnane X receptor (PXR), VDR, and TGR5. Each BA has unique receptor selectivity and affinity, with CDCA being the most potent natural activator of FXR, and UDCA acting as an antagonist of FXR. TGR5 is highly sensitive to conju- gated BAs, whereas hydrophobic free BAs preferentially bind to nuclear receptors such as PXR and VDR. Our study also found that bear bile TAAs have remarkable antipyretic effects. Taurine, a component of TAAs, has been reported to be beneficial for treating and preventing diseases associated with mitochondrial defects, such as aging, metabolic syndrome, cardiovascular disease, and neurological disorders. Therefore, to achieve the equivalent effects of bear bile, all seven major BAs mentioned above, as well as TAAs, must be included in the formulation of ABB.
3.2.1. Synthesis of BAs based on enzyme engineering
Commercially available BAs such as UDCA, CDCA, and cholic acid (CA) are mainly produced by extracting and chemically con- verting bile from chicken, cattle, and pigs. Unfortunately, this pro- cess is labor-intensive and environmentally unfriendly. In recent years, enzymatic synthesis of BAs has been developed, with UDCA biosynthesis using NAD/NADP-dependent 7b-HSDH being applied commercially.
In this study, an enzymatic platform utilizing three hydroxys- teroid dehydrogenases was developed to achieve the high- efficiency preparation of all major BAs (contents above 0.5%) in bear bile (Fig. 2). Chicken bile pastes were used as the starting material, with CA and CDCA, the two major constituents of chicken bile, being prepared by saponification, followed by extraction and crystallization. The key intermediates 7-ketolithocholic acid
(7KLCA) and 7a-hydroxy-3-oxo-5b-cholanoate (3KDCA) were prepared from CDCA under the catalysis of 7a-HSDH and 3a-HSDH, respectively.
Next, CDCA was directly converted to UDCA, catalyzed by 7a-and 7b-HSDH in a double-enzyme-coupled system. Free BAs such as CA, CDCA, 7KLCA, UDCA, and 3KDCA were conjugated with tau- rine to produce TCA, TCDCA, T7KLCA, TUDCA, and T3KDCA, respectively. Finally, all seven BAs in bear bile (with a content above 0.5%) were prepared on a kilogram scale for the first time, setting the stage for the mass production of ABB. Data are provided in Section S1.1 in Appendix A.
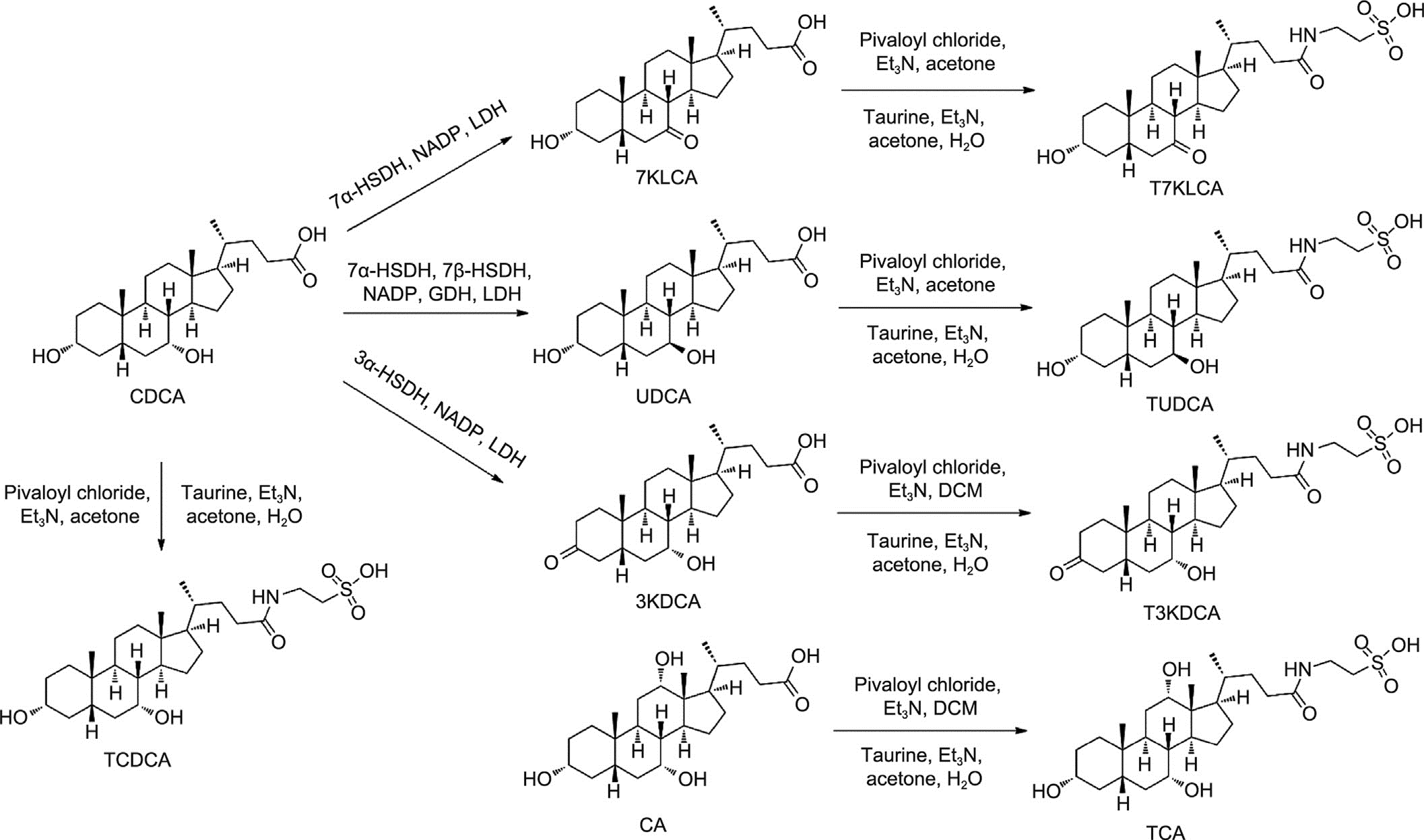
Fig. 2. Synthesis of seven BAs in bear bile. 7KLCA: 7-ketolithocholic acid; 3KDCA: 7a-hydroxy-3-oxo-5b-cholanoate; DCM: dichloromethane; Et3N: triethylamine.
3.2.2. Seeking alternatives to bear bile TAAs
To explore potential alternatives to bear bile TAAs, the pharma- cological effects of TAAs from various livestock or poultry were investigated. TAAs were prepared from the bile pulp of chickens, ducks, geese, and cattle, respectively. Efficacy evaluation results (Table S15 in Appendix A) showed that TAAs prepared from the bile pastes of these animals had antipyretic effects similar to those of bear bile TAAs.
However, it is noteworthy that chicken BAs mainly comprised TCA and TCDCA, which are also present in bear bile, while the duck and cattle bile contained significant amounts of allocholic acid and glycocholic acid, respectively, which do not exist in bear bile. Chicken bile TAAs contain essentially the same amino acids found in bear bile and are thus more suitable as an alternative to bear bile TAAs, considering the efficacy, chemical similarity, and raw mate- rial availability.
3.2.3. Formula optimization of ABB
Based on the data obtained from a large sample size, the total BA (conjugated and free) content of bear bile was found to be relatively stable (relative standard deviation (RSD) = 7.5%), with minor com- ponents such as TCA, T7KLCA, T3KDCA, taurine, and TAAs having lit- tle effect on its fluctuation. Therefore, the levels of total bile acids (TBAs) and minor components in the ABB were maintained within a range consistent with high-quality natural bear bile.
To investigate the three major variables that could potentially influence the overall efficacy of ABB, the ratio of TUDCA to TCDCA, that of UDCA to CDCA, and the relative abundance of conjugated versus free BAs were examined. Orthogonal experiments were designed to optimize the composition ratio of the ABB. The pharmacological effects of nine formulations on the multi-model evaluation system were compared (Tables S16–S19 in Appendix A). The final formulation was optimized by fully considering both the pharmacological effects and the costs. Its composition was highly consistent with natural bear bile in terms of type, content, and proportion of chemical components such as BAs, amino acids, and inorganic elements.
3.2.4. Pharmacodynamic consistency of ABB and DBB
Three batches of ABB were prepared, each on a 10 kg scale, and their quality was found to be stable, indicating that the preparation process is suitable for industrial production. A systematic study was conducted to compare the medicinal effects of ABB and DBB (Tables S20–S39 in Appendix A).
Since ancient times, bear bile has been used to treat fever, which is a mechanism by which the body defends against microorganisms and inanimate substances. Pyrogens are the primary factors responsible for inducing a febrile response. Among them, yeast and LPS from the outer membrane of Gram-negative bacterial cells are potent inducers of fever, infectious shock, systemic tissue dam- age, and death in severe cases [41]. In the yeast-induced fever model (Figs. 3(a) and (b)), ABB (50, 100, and 300 mg∙kg—1) showed effects similar to those of the positive control, aspirin (300 mg∙kg—1), and significantly reduced hyperthermia in rats from 4 to 8 h (P < 0.05). In the LPS-induced fever model (Figs. 3(c) and (d)), ABB at a dose of 50 mg∙kg—1 significantly inhibited hyperthermia (P < 0.01) from 4 to 6 h after modeling, while ABB at 100 mg∙kg—1 (P < 0.05) and aspirin at 300 mg∙kg—1 (P < 0.001) displayed significant changes in hyperthermia from 4 to 8 h.
Bear bile is still commonly used to reduce swelling and alleviate
pain. To assess the analgesic benefits of ABB and DBB, two different models were established. In the hot plate test, aspirin, gabapentin, and ABB in each dose group increased the pain threshold of ICR mice to some extent. The analgesic effects of gabapentin (300 mg∙kg—1) and ABB at the same dose were statistically different from those of the normal control group (P < 0.05), and it is worth noting that ABB was superior to gabapentin in improving the pain threshold (64.5% vs 34.5%) (Table S24). Basically, in both the hot plate and acetic acid writhing tests, the analgesic effects of ABB were comparable to those of DBB in mice (Figs. 3(e)–(g)).
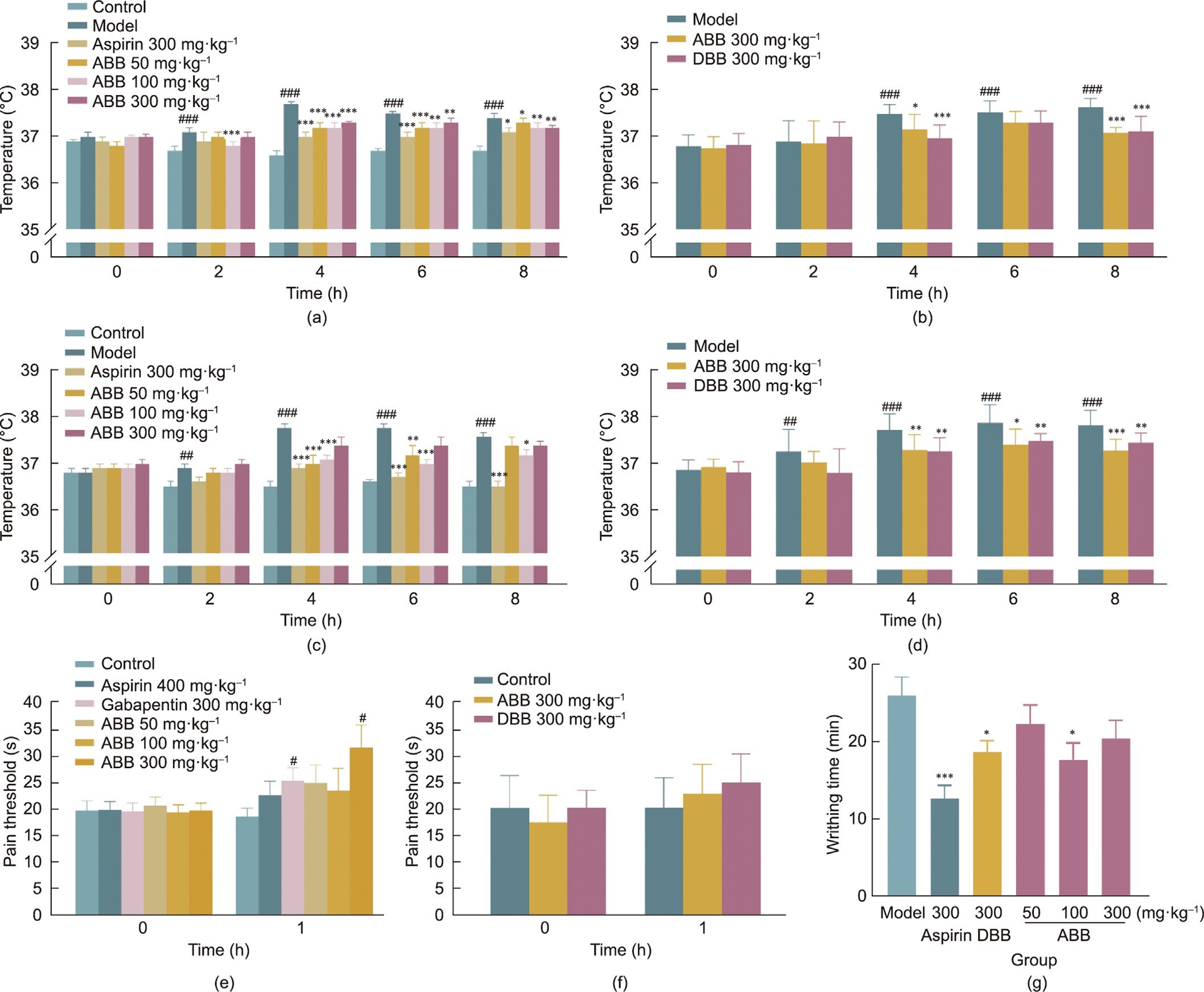
Fig. 3. (a–d) Pharmacological (antipyretic and analgesic) efficacy of ABB and DBB. Rat fever induced by (a, b) yeast and (c, d) LPS. (e, f) Hot plate test. (g) Acetic acid-induced writhing. ##P < 0.01, ###P < 0.001 vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model.
According to a report by Brevini et al. [19], UDCA may poten- tially prevent COVID-19 via reducing the sensitivity of SARS-CoV- 2 infection by down-regulating ACE2. However, the data from the present study suggest that the oral administration (after 2 or 24 h of infection) of ABB or DBB demonstrated only a marginal antiviral effect against H1N1 influenza virus in mice, which was inferior to the effect of the positive control drug Tamiflu (Figs. 4(a) and (b)). Given the fact that influenza viruses and coronaviruses belong to different virus families, and have significant differences in their genomic structures and morphological features, further research is still needed to determine whether bear bile has similar effects on coronaviruses.
PTZ induces seizures by binding to receptors in the brain and
increasing the release of glutamatergic neurotransmitters, leading to overactivity of excitatory neurons and ultimately triggering epileptic episodes. In a single-dose experiment (Figs. 4(c)–(e)), CBZ, a positive drug, significantly delayed the onset of epilepsy and decreased seizure scores (P < 0.05). However, neither the ABB nor the DBB demonstrated any significant effect on delaying the onset of epilepsy. Nevertheless, groups receiving ABB powder at doses of 50 and 100 mg∙kg—1, as well as DBB, showed a signifi- cant reduction in seizure scores (P < 0.05). After being adminis- tered for seven consecutive times (Figs. 4(f)–(h)), both CBZ and ABB were able to significantly delay the onset of class III epilepsy (P < 0.05) (Fig. 4(g)), ABB at doses of 100 and 300 mg∙kg—1 also delayed the onset of epilepsies below class III (Fig. 4(f)), although it showed an improving trend, neither had a significant impact on the kindling scores.
In TCM, bear bile is classified under the liver and gallbladder meridians, and ‘‘regulating the liver” is considered to be one of its core functions. This aligns with the modern understanding of using BAs to treat liver and gallbladder diseases. To evaluate the hepato-protective and cholagogic effects of bear bile, three differ- ent liver injury inducers (CCl4, Con-A, and ANIT) were employed to model liver damage caused by distinct mechanisms.
In the CCl4-induced liver injury model, a classic chemical injury model, bicyclol (as a positive control) significantly reduced the serum ALT levels, while showing no significant difference in AST levels. In addition, both the DBB and ABB effectively reduced the serum ALT levels in mice with liver injury at a dose of 300 mg∙kg—1. ABB at the same dose also significantly decreased the AST levels (P < 0.05) (Figs. 5(a) and (b)).
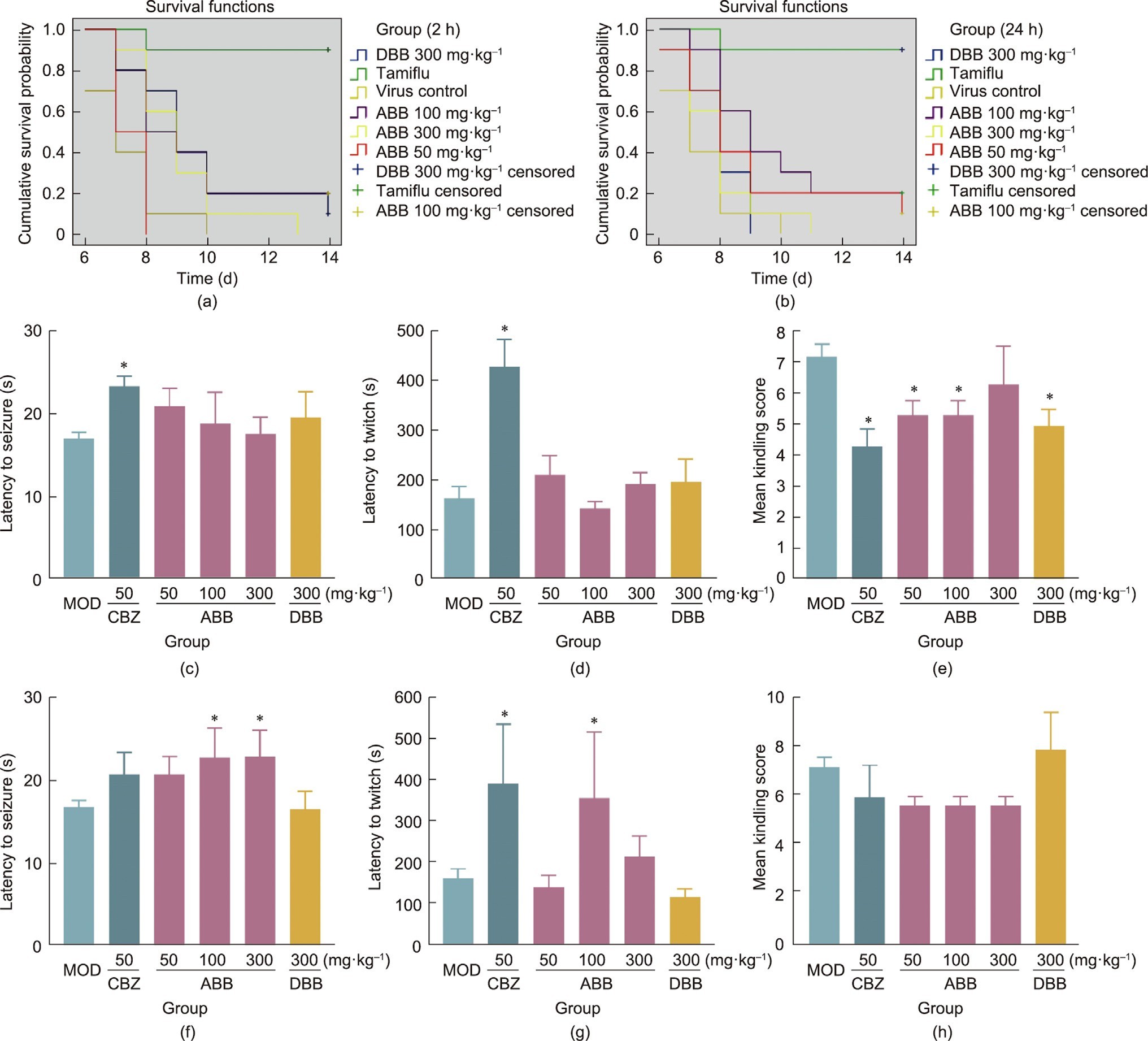
Fig. 4. (a, b) Survival map of mice administered an H1N1 viral infection after (a) 2 or (b) 24 h. (c–h) Effects of (c–e) single or (f–h) seven continuous administrations on PTZ- induced seizure. *P < 0.05 vs MOD: model.
In a mouse model of immunological liver injury induced by Con-A, the administration of DBB powder at various doses resulted in significant decrease in serum ALT and AST levels (P < 0.05). However, due to substantial intra-group variability, no clear dose–response relationship was observed across different dosage groups. Notably, at a dose of 300 mg∙kg—1, the ABB powder significantly reduced the serum ALT and AST levels in mice with liver injury (P < 0.01). In addition, at a dose of 200 mg·kg—1, bicyclol was found to effectively reduce the serum ALT levels, although it had no significant effect on AST levels (Figs. 5(c) and (d)).
ANIT can interfere with the physiological process of bile excretion, resulting in cholestasis and impaired BA synthesis. Such pathological changes can lead to hepatocellular injury. In the ANIT-induced liver injury model, GRZ, a positive drug, significantly reduced the levels of ALT, AST, and TBIL in the serum of mice with cholestatic jaundice (P < 0.05). ABB (50–300 mg∙kg—1) and DBB (100–300 mg∙kg—1) were shown to significantly decrease the serum levels of ALT in model mice. Similarly, ABB (50–100 mg∙kg—1) and DBB (100–300 mg∙kg—1) demonstrated significant decrease in the serum levels of AST and TBIL in model mice (P < 0.05) (Figs. 5(e)–(g)).
In TCM theory, high levels of blood sugar and lipids are com- monly attributed to excessive heat in the body. Bear bile, on the other hand, is regarded as having cooling and heat-clearing effects. To evaluate the hypoglycemic and lipid-lowering proper- ties of bear bile, KKAy mice—a commonly utilized model of type
2 diabetes in preclinical research—were used. The results indi- cated that the administration of ABB for four consecutive days resulted in a significant reduction in TG levels in both the 100 and 300 mg∙kg—1 dose groups, with only the 300 mg∙kg—1 dose group showing a significant decrease in fasting blood glucose (FBG). While all the groups exhibited a trend toward decreased CHO, no statistical difference was observed. However, after the administration of DBB, both the 100 and 300 mg∙kg—1 dose groups showed significant reductions in FBG, TG, and CHO levels (Figs. 5(h)–(j)).
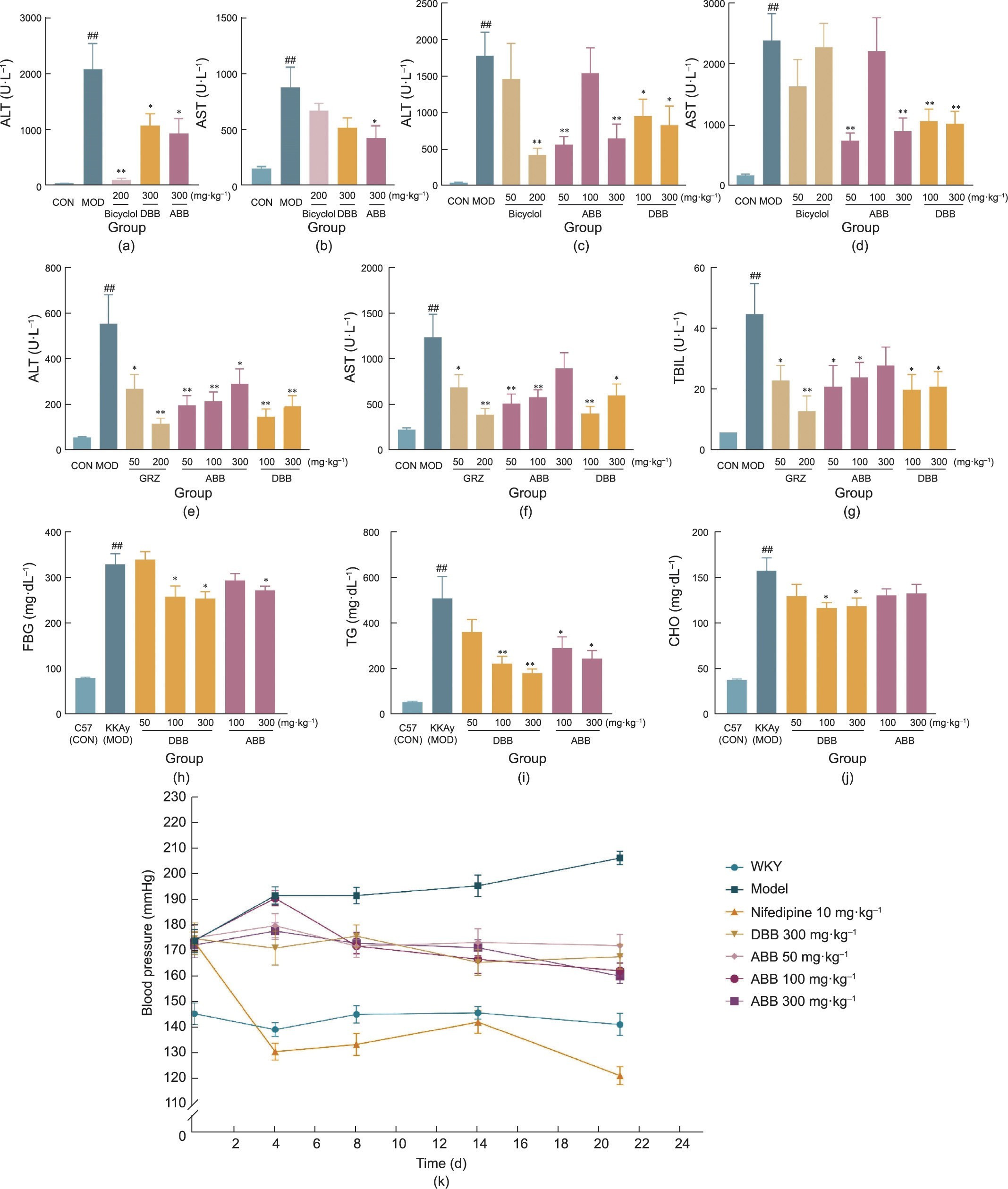
Fig. 5. Pharmacological (hepatoprotective, cholagogic, hypoglycemic, hypolipidemic, and blood pressure regulation) efficacy of ABB and DBB. (a–d) Protective effects on (a, b) CCl4-induced chemical and (c, d) Con-A-induced immune liver injury in mice; (e–g) protective effects on ANIT-induced cholestatic jaundice; (h–j) effects of multiple oral administrations on (h) FBG, (i) TG, and (j) CHO in KKAy mice; (k) alleviation of spontaneous hypertension of SHR rats. ##P < 0.01 vs control; *P < 0.05, **P < 0.01 vs model. CON: control.
Bear bile is widely utilized in TCM for treating hypertension and related disorders. The results showed that, from seven weeks of age, the average blood pressure of SHR rats had already reached (160.0 ± 1.9) mmHg (1 mmHg = 133.3224 Pa). Compared with WKY rats at the same age, the SHR rats had significantly increased blood pressure (P < 0.05). Beginning at eight weeks of age, the positive drug nifedipine, DBB, and different doses of ABB powder were orally administered daily for 21 consecutive days. On days 0, 4, 8, 14, and 21 of medication, all group animals’ body weight, blood pressure, and heart rate were monitored. Nifedipine effectively lowered the model rats’ blood pressure from day 4 of medication (P < 0.05). The 300 mg∙kg—1 dose of DBB and the 50–300 mg∙kg—1 dose groups of ABB all exhibited significant and sustained inhibi- tory effects on the model rats’ blood pressure increase (P < 0.05), with the efficacy of ABB being essentially equivalent to that of DBB (Fig. 5(k)).
To further elaborate the pharmacological consistency of the ABB and DBB, a comparative study was conducted, based on the analy- sis of the experimental data, which involved grading the efficacy of each model separately. Table 2 provides an overview of the grading results, which demonstrate that both ABB and DBB have significant effects on several models, including antipyretic, analgesic, hepato- protective, cholagogic, hypoglycemic, lipid-lowering (TG, CHO), and blood pressure-regulating (antihypertensive) models. In addi- tion, under the experimental conditions of this study, neither ABB nor DBB demonstrated significant pharmacological effects in antibacterial and anticerebral ischemic models, although there were certain trends of activity, none reached statistical signifi- cance. These findings further support the traditional applications of bear bile in Chinese medicine theory and provide scientific evi- dence for its clinical use. Moreover, these discoveries aid in a better understanding of the active constituents and mechanism of action of bear bile, thereby enhancing comprehension of its pharmacolog- ical properties and wide-ranging applications in the field of Chinese medicine.
We conducted a comprehensive evaluation of the pharmacological consistency of ABB and DBB both in vitro and in vivo. The results provided robust evidence demonstrating that ABB is comparable to—or even superior to—DBB in many patholog- ical models, despite slight inferiority in certain aspects. Overall, ABB can be considered an ideal alternative to DBB. Further clinical comparative studies between ABB and DBB will be conducted to confirm their equivalence.
3.2.5. Pharmacokinetics of ABB
Oral administration of ABB was carried out in SD rats and beagle dogs, with plasma levels of UDCA, CDCA, TUDCA, and TCDCA deter- mined by means of high-performance liquid chromatography- tandem mass spectrometry (HPLC-MS/MS). Fig. 6 presents the time–concentration curves of CDCA, TCDCA, UDCA, and TUDCA in SD rats, respectively; their pharmacokinetic parameters are pro- vided in Appendix A. In male SD rats, the area under curve (AUC)(0–12h) of CDCA and UDCA increased linearly with increasing doses, while that of TCDCA and TUDCA increased nonlinearly with increasing doses. The absorption of CDCA was rapid, with a time to peak drug concentration (Tmax) ranging from (5.00 ± 0) to (9.00 ± 2.24) min and a maximum concentration (Cmax) from (7.97 ± 2.33)
to (12.40 ± 6.20) lg∙mL—1. In comparison, the Tmax of UDCA ranged from (128.00 ± 162.97) to (170.00 ± 159.50) min, with a Cmax of (2.86 ± 1.59) to (5.49 ± 2.14) lg∙mL—1. Compared with the free BAs, TCDCA and TUDCA showed smoother curves, a smaller Cmax, and multiple peaks indicative of enterohepatic circulation.
The Tmax of TCDCA was much longer than that of its free form, CDCA, ranging from (127.50 ± 131.98) to (260.00 ± 217.14) min, with a Cmax of (204.65 ± 136.62) to (631.57 ± 373.86) ng∙mL—1, indicating that the conjugated BAs are transported at a slower rate than the free BAs. After oral administration (100 mg∙kg—1) of ABB to female rats, the AUC(0–12h) of TCDCA and TUDCA were compara- ble to those of male rats. However, the AUC(0–12h) of CDCA and UDCA were significantly lower than those of male rats, suggesting that gender differences in metabolism may exist.
Table 2. Evaluation of consistency between ABB and DBBa.
| Samples | Antipyretic | Analgesic | Hepatoprotective | Cholagogic (TBIL) | Hypoglycemic | Hypolipidemic | Antihypertensive | Antiviral | Antibacterial | Anti-cerebral ischemia | Anticonvulsant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeast-induced | LPS-induced | Hot plate | Acetic acid writhing | CCl4-induced | Con-A-induced | Cholestasis hepatitis | TG | CHO | in vitro | in vivo | |||||||
| ABBb | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 1 |
| DBBb | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 0 |
- a
-
Significant difference in activity compared with the model group was noted for 2 points, only a trend in activity compared with the model group was noted for 1 point, and no trend in activity compared with the model group was noted for 0 points.
- b
-
ABB pilot batch (Batch No. 161101). Reference DBB provided by China National Traditional Chinese Medicine Corporation.
3.2.6. Preclinical toxicological assessment of ABB
To provide a reference for clinical trials, the acute toxicity (sin- gle oral administration) of ABB was assessed in SD rats and beagle dogs to observe any possible toxic reactions and to compare them with those of marketed DBB powder. The general condition of each group was observed for 14 consecutive days after administration, and no obvious abnormalities were observed in the weight, food intake, blood pressure, electrocardiogram, hematology, blood bio- chemistry, and urine routine test of each group.
In the long-term toxicity assessment, the SD rats and beagle dogs were gavaged for 28 consecutive days with various doses of ABB powder and marketed DBB powder. The non-toxic response dose (NOAEL) of the ABB was 1 g∙kg—1, with no significant abnormal changes observed during the observation period. However, the male rats in the 1000 mg∙kg—1 group of marketed DBB powder showed a decrease in body weight, kidney organ weight, and kidney–brain ratio. An elevated ALT level was also observed in the beagle dogs in the high-dose group (1000 mg∙kg—1) of the marketed DBB powder. Elevated TBA was found in all groups of dogs with both DBB and ABB powder, mostly due to pharmacology-related changes.
The results of the study on reproductive toxicity revealed that
1 g∙kg—1 was the NOAEL for reproductive function and early embryonic development in female rats. Furthermore, the Ames, micronucleus, and chromosome aberration tests demonstrated negative results, indicating a satisfactory safety profile in terms of mutagenicity and genotoxicity for both the ABB and DBB.
3.2.7. Phase I clinical trial on tolerance in healthy volunteers
A randomized, double-blind, single-center trial was conducted to assess the tolerance of ABB in healthy volunteers. The study included both single and continuous dosing experiments, with positive drug (DBB) and placebo controls. Vital signs, physical examination, clinical symptoms, and physical and chemical examinations were used to evaluate the clinical safety of the drugs.
Examinations of various bodily systems, including the circula- tory, respiratory, digestive, urinary, and hematological systems, were conducted to assess the effects of the drugs on the healthy volunteers. A total of 134 healthy volunteers participated in the tolerance study, with 86 receiving a single dose and 48 receiving continuous doses. The distribution and completion of volunteers in each group are detailed in Table 3. No serious adverse events (SAEs) or unintended adverse reactions (SUSARs) were observed for both ABB and DBB within the dosing range of 0.2–2.0 g∙d—1.
The main adverse reactions for both drugs were gastrointestinal system reactions such as abdominal pain and nausea, as well as hepatic biochemical abnormalities such as increased ALT, AST, or c-glutamyl transferase (GGT), which mostly occurred at doses exceeding the clinical dosing range 1.2–2.0 g·d–1. Within the clin-
ically recommended dose 0.2–0.8 g·d–1, one adverse reaction occurred with the test drug in the form of abdominal pain, which was resolved without intervention after defecation, and one adverse reaction occurred with the positive control drug in the form of hemorrhoidal bleeding, which was also resolved without intervention.
No significant differences were observed in the type and extent of adverse reactions between the ABB and DBB groups. However, since the study population consisted of healthy subjects with a limited sample size, subsequent clinical trials with a larger number of patients are required to further evaluate the safety and efficacy of ABB and provide a solid foundation for its large-scale clinical application. Additional data, including comprehensive findings from the Phase II clinical trial, will be meticulously documented and published in subsequent articles.
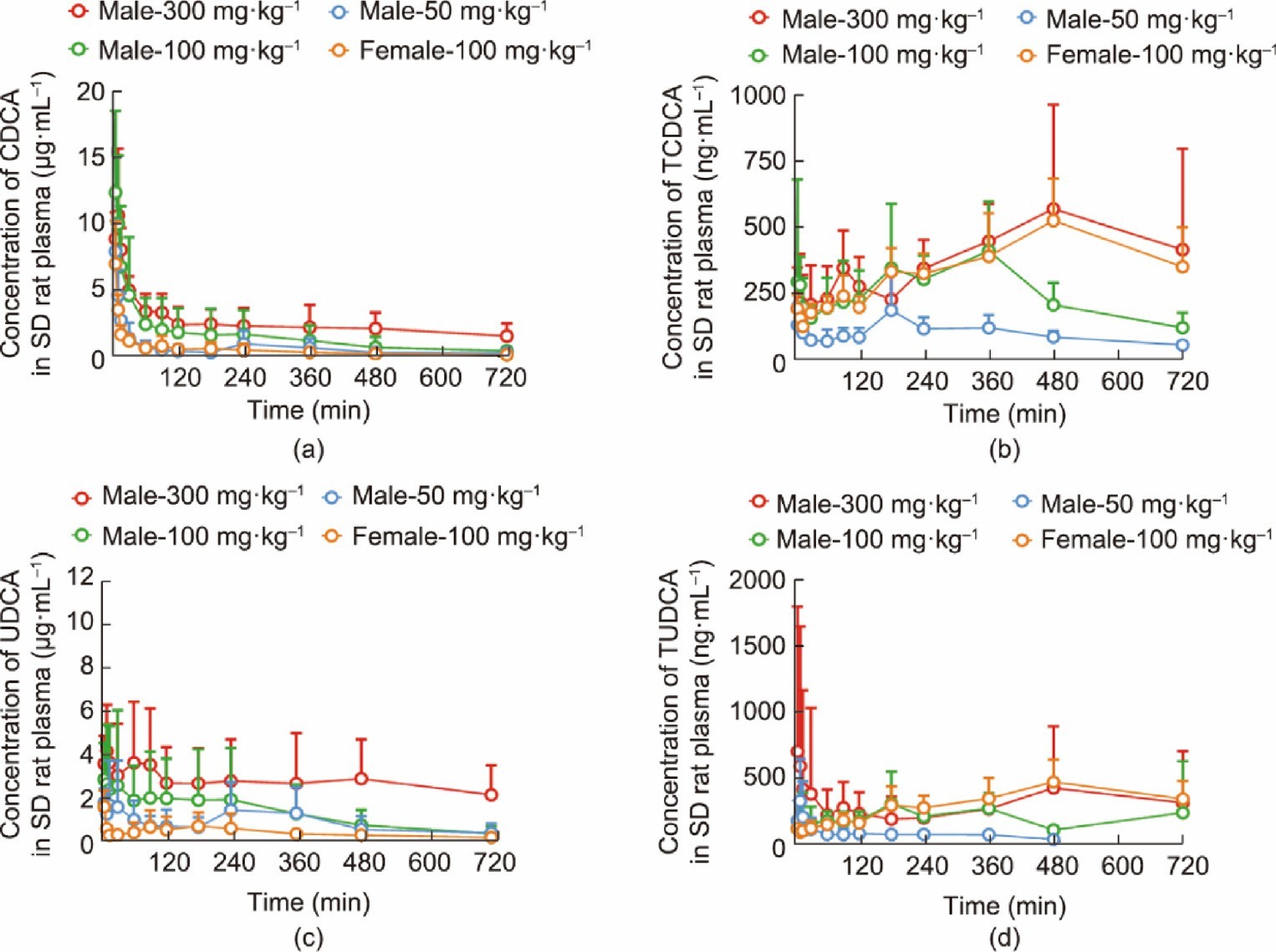
Fig. 6. Drug time–concentration curves for (a) CDCA, (b) TCDCA, (c) UDCA, and (d) TUDCA after oral administration of low, medium, and high doses of ABB in male SD rats and medium doses in female SD rats (mean standard deviation, n = 5).
Table 3
Enrollment of participants and status of completion.
Group Number Single dose number Continuous dose numbera
| 0.2 g∙d–1 | 0.4 g∙d–1 | 0.8 g∙d–1 | 1.2 g∙d–1 | 1.6 g∙d–1 | 2.0 g∙d–1 | 1.6 g∙d–1 | 1.2 g∙d–1 | ||||
| ABB | 48 | 2 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | ||
| DBB | 48 | 2 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | ||
| Placebo | 38 | 2 | 4 | 4 | 4 | 4 | 4 | 8 | 8 | ||
| Participant | 134 | 6 | 16 | 16 | 16 | 16 | 16 | 24 | 24 | ||
| Dropout | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Excluded | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Completion | 132 | 6 | 16 | 16 | 16 | 16 | 16 | 23 | 23 | ||
a Continuous dosing for 14 d.
4. Conclusions
The study of bear bile alternatives presents numerous scientific challenges in various domains including resources, chemistry, biology, medicine, pharmacology, and TCM, as bear bile has com- plex mechanisms of action and highly variable chemical compositions. Addressing these challenges requires novel approaches that involve new methodologies and interdisciplinary collaborations.
In this study, we offered a fresh approach to creating ABB by interpreting the scientific basis of bear bile and integrating multiple technologies from different disciplines. We developed a com- prehensive efficacy assessment system that describes the therapeutic effects of bear bile in a way that reflects the clinical experience of TCM and bridges the gap between traditional and modern medical terminology and philosophy. Using chemical syn- thesis and enzyme engineering technologies, we produced the therapeutic compounds present in bear bile in an efficient and environmentally friendly manner. This allowed for the bulk preparation of these therapeutic components and the systematic screening of ABB formulations.
The resulting ABB not only closely resembles natural bear bile in its composition, but also offers advantages such as consistent product quality, affordability, availability of raw materials, and independence from threatened or wild resources. Comprehensive preclinical efficacy evaluations have demonstrated the equivalence of the therapeutic effects of the ABB and those of commercially available DBB. Furthermore, preclinical pharmacological assessment and a clinical trial on healthy volunteers demonstrated that the ABB’s effectiveness is on par with that of the currently used DBB, and the toxicological trial data support a favorable safety profile.
The creation of ABB will resolve the bear bile supply issue and provide high-quality alternatives for use in medicine. It will also strengthen the integrity and sustainability of TCM, while supporting the protection of endangered bears and their welfare. This innovative strategy can serve as a new research paradigm for the development of alternatives for other endangered TCMs.
Source: ScienceDirect